Explain How a Hemiacetal Compound Differs From a Hemiketal Compound
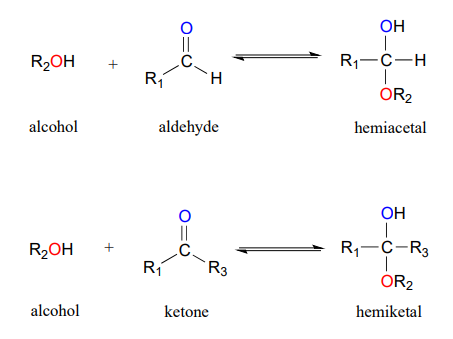
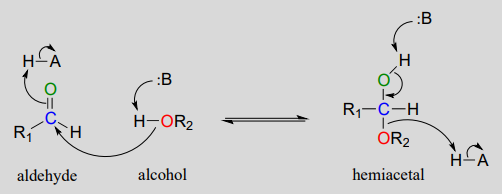
A hemiacetal is an alcohol and ether attached to the same carbon. The Greek prefix hèmi means half refers to the fact that a single alcohol has been added to the carbonyl group.
10 3 Hemiacetals Hemiketals And Hydrates Chemistry Libretexts
Explain what the difference is between aniline and anilinium chloride.

. In solution 99 of glucose exists in the cyclic hemiacetal form and only 1 of glucose exists in the open form. The acetal and hemiacetal carbon is actually the central carbon derived from the carbonyl group and these forms only exist in cyclic forms ie. Start your trial now.
The key difference between Hemiacetal and Hemiketal is that hemiacetal is formed via the reaction between an alcohol and an aldehyde whereas a hemiketal is formed via the reaction between an alcohol and a ketone. Any of a class of compounds derived from aldehydes by adding an alcohol to the carbonyl. Difference between acetals and ketals being one hydrogen atom on the carbon attached to the two oxygen and hemiacetalhemiketal being one -OH group.
A cyclic form of glucose is a hemiacetal compound. Here the R groups can be organic fragments or hydrogen while the R groups must be organic fragments not hydrogen. Hemiacetal is an intermediate formed during the formation of acetal.
-the carbonyl carbon becomes chiral in the process of forming hemiacetal or hemiketal. Acetal and hemiacetals are recognized as functional groups. October 12 2016 Posted by Abey SD.
Explain the difference between esterification and glycoside formation. Hemiacetal and hemiketal are organic compounds that can be observed as hybrid molecules containing two functional groups in the same. Hemiacetal Hemiketal Acetal Ketal Other Answer Bank CH3 H3C OH HC CH OH OCH CH COCH CHCH OH OCH CH.
The mechanism of the reaction is similar to what we learned in the acid-catalyzed hydration of. Other names are formed by. There are also N- S- and C-glycosidic bonds.
Acetals and Hemiacetals with Practice Problems - Chemistry Steps. Acetals are tetrahedral compounds where two alkoxy OR groups are bonded to the central carbon atom. VIodoform test to.
Acetal and hemiacetal are groups of atoms considered as functional groups. Compounds with the general structure R R X C O H O R X have the class name hemiacetals. In hemiacetals one of the OR groups in acetals is replaced by a OH group.
The bond formed between the hemiacetal or hemiketal on the first carbohydrate and the hydroxyl group on the second molecule is an O-glycosidic bond. They are named substitutively as alkoxy alkyloxy aryloxy etc. The fourth bonding position is occupied by hydrogen.
Cr 2 O 7 2-H Tollens Fehlings and Schiffs reagents to differentiate aldehydes from ketones. Intramolecular hemiacetal and hemiketal formation is commonly encountered in sugar chemistry. Aldehydes and ketones react with alcohols under acidic conditions to form acetals.
As an example the drug Amlodipine marketed under the name Norvasc. However many people use the term acetal for both ketals and acetals and also use the term hemiacetal for both hemiketals and. Is anilinium chloride C6H5NH3 Cl- a good nucleophile.
This is the key difference between acetal and hemiacetal. The hemiacetalhemiketal initially formed is converted. In a hemiketal neither R-group can be a hydrogen.
There is a slight difference between their chemical structures. An acetal is a functional group with the connectivity R2C2. The sp3 hybridized carbon atom that is attached to the four different.
Science Chemistry QA Library lassify these structures as hemiacetal hemiketal acetal ketal or other. Solution for Classify the following compound. The equilibrium between the carbonyl forms of aldehydes or ketones and their associated acetalhemiacetal or ketalhemiketal forms also plays a critical role during the bodys metabolism of xenobiotics drugs.
Acetals contain two OR groups one R group and a H atom. The compound is Ketal right. The main difference between acetal and hemiacetal is that acetals contain two OR groups whereas hemiacetals contain one OR and one OH group.
- similar to starch but has more alpha-16 glycosidic bonds which makes it a highly branched compound. Similarly fructose is a hemiketal compound. A hemiacetal is derived from an aldehyde.
An electron pair acceptor. The main difference between acetal and hemiacetal is that acetals contain two -OR groups whereas hemiacetals contain one -OR and one. Explain the preparation of carbonyl compounds through.
Acetals and hemiacetals are two functional groups which are most commonly found in natural products. Derivatives of an appropriate hydroxy parent compound such as an alcohol. The hemiacetal is really the combination of two functional groups.
The two R groups can be equivalent to each other or not. Acetals hemiacetals ketals and hemiketals in drug metabolism. Meaning pronunciation translations and examples.
Hemiacetals are formed from. According to the IUPAC definition in R 1 R 2 C OHOR R 1 and R 2 may or may not be a hydrogen. Glycosidic bonds are labeled according to the identity of the atom on the second carbohydrate or the functional group.
Start studying Chemistry Exam 2- Hemiacetals and Acetals. Classify the following compound. A hemiacetal is a carbon connected to two oxygen atoms where one oxygen is an alcohol OH and the other is an ether OR.
Learn vocabulary terms and more with flashcards games and other study tools. The term Hemiacetal derives from the Greek word hemi which means half. Hemiacetals on the other hand are reaction intermediate compounds having relatively less stability.
Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon but have. These names are the preferred IUPAC names. In fact the sugar glucose may be the most commonly known hemiacetal.
Is a ketal you are correct. Want to see the full answer. An electron pair donor.
IOzonolysis of alkene iiFriedel-Crafts acylation to produce aromatic ketone CARBONYL COMPOUNDS C O R R C O H R Explain the chemical properties with reference to. Just to give you an example. Hemiketals are regarded as hemiacetals where none of the R-groups are H and are therefore a subclass of the hemiacetals.
Honey bee indicating honey is sweet because it contains glucose and fructose.
What Is A Hemiacetal Functional Group Quora
Difference Between Acetal And Hemiacetal Definition Formation
10 3 Hemiacetals Hemiketals And Hydrates Chemistry Libretexts

Glucose Versus Fructose Chemical Structure A The Hemiacetal Group Of Download Scientific Diagram
No comments for "Explain How a Hemiacetal Compound Differs From a Hemiketal Compound"
Post a Comment